Why It Is Difficult To Find the Perfect Clean Room Wipe And How MiraWIPE Scores high…Here’s A Simple Analysis
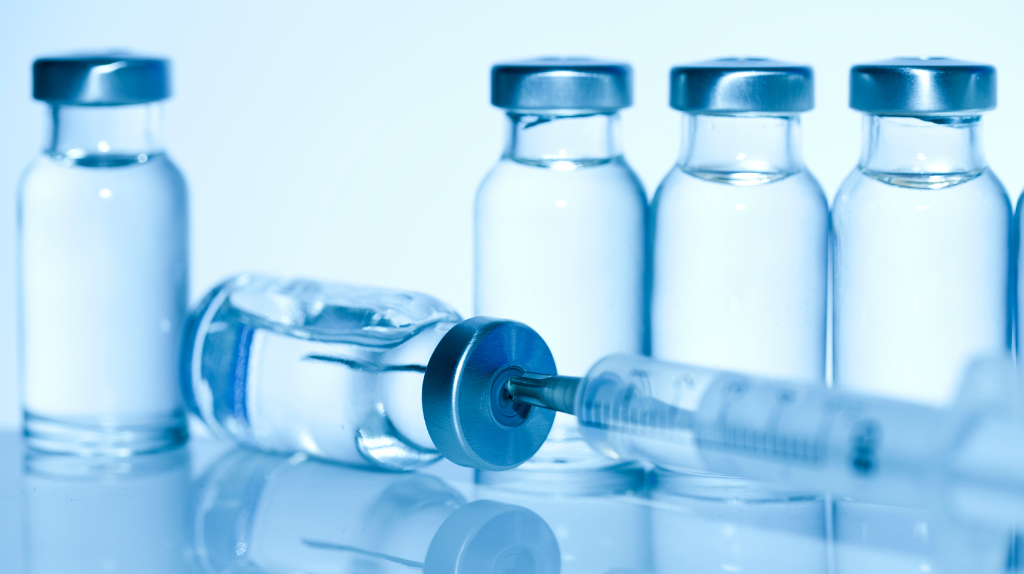
In critical fill-finish sterile areas, even the smallest particles and fibers can cause significant contamination issues endangering the finished injectable product for usage, leading to high rejection rates and increased costs. Choosing the right wipe thus becomes critical to ensure surfaces remain uncontaminated and finished products are not compromised. Which is why, Clean Room wipes […]
Eliminating Residual Drug Contaminants: How the Sahara+ System Maintains Clean Surfaces while minimizing risk of cross over contamination in Oral and Solid Dosage Manufacturing

Maintaining an immaculate environment is essential in oral and solid dosage manufacturing. However, even the most stringent cleaning protocols can struggle against stubborn drug residues deeply entrenched in the grain of the equipment surfaces. Traditional methods often resort to harsh chemicals or abrasive techniques, risking damage to delicate equipment and compromising product integrity. Introducing the […]
Mastering Corrosion: The Role of Diamond ScrubPADS and PneuSCRUB in Stainless Steel Equipment Restoration for Oral and Solid Dosage Manufacturing

Image Credit: DALL.E In the oral and solid dosage manufacturing, maintaining impeccable facility standards is paramount. However, even with meticulous cleaning protocols, the persistent challenge of corrosion can occasionally emerge, threatening the integrity of stainless steel equipment. Corrosion not only compromises visual aesthetics but also creates potential havens for contaminants, thereby jeopardizing both product quality […]
4 Key Insights on Facility Maintenance and Cleaning from the PDA Conference 2024, Hyderabad

Image Credit: DALL.E In our previous blog post, we elaborated on some of the frequently asked questions (FAQs) on Leak Testing/Headspace Analysis that our team answered at the recently held PDA Conference in Hyderabad. In this blog post, let’s uncover some key questions on facility maintenance and cleaning that we were asked by leading Injectable/OSD […]
6 Key Insights on Leak Testing/Headspace Analysis from the PDA Conference 2024, Hyderabad

The recently held three-day Parenteral Drug Association (PDA) India Chapter’s Annual Conference 2024 in Hyderabad witnessed a strong turnout of over 600 experts from the pharmaceutical industry. As one of the leading subject matter experts in CCIT, Headspace Analysis, Clean Room Contamination Control and Facility Maintenance solutions, we were invited by the organisers to exhibit […]
The Critical Role of Adopting a Simple Cleaning Step in ISO 5 Clean Rooms

Image credit: DALL.E One among these is the deep cleaning, abrasion-resistant MiraWIPE which is an indispensable tool in maintaining the stringent sterility standards required in ISO 5 Clean Rooms. Navigating the Cleaning Challenge in ISO 5 Pharmaceutical Clean Rooms The cleaning and maintenance of ISO 5 pharmaceutical Clean Rooms represents a multifaceted challenge, one that […]
Combating Corrosion in Clean Rooms: Materials, Causes, and Innovative Solutions
Image credit: DALL.E Clean Rooms play a pivotal role in maintaining product integrity and ensuring compliance with stringent pharma regulatory standards. However, these controlled environments are constantly threatened by an insidious foe: corrosion. This silent destroyer can compromise equipment, contaminate products, and lead to expensive product recalls, downtime and frequent repairs. Let’s understand everything there […]
A Novel Solution for On-Press Mold Cleaning in Clean Rooms

Image credit: DALL.E When it comes to pharmaceutical injection moldings, the critical importance of cleanliness and precision cannot be overstated. Ensuring effective mold cleaning is a fundamental component for adhering to the exacting quality standards required in Clean Room environments. Let’s look at a novel approach that is redefining the methodology for on-press mold cleaning in […]
Managing FM-Related CAPAs in the Medical Device Industry

Understanding FM-Related CAPAs Grover Holdings recognizes the gravity of this issue and understands the importance of mitigating these risks. CAPAs serve as the guiding framework for rectifying and preventing FM-related issues. We assist medical device companies in eliminating FM-related challenges. As the medical device industry grows increasingly complex, our expertise and innovative strategies empower us […]
Enhancing Clean Room Contamination Control: Beyond Regular Visual Inspection

Image credits: Foamtec When it comes to the complex world of manufacturing sterile pharmaceuticals, maintaining contamination control in Clean Room environments becomes paramount. The Common Challenge in Validating Clean Room Surfaces Good Manufacturing Practice (GMP) procedures mandate that operators frequently sanitize the work surfaces at the beginning and end of their shifts, as well as […]

