Are pH Buffer Residues Stealthily Eroding Your Stainless-Steel Equipment?

In facilities producing biologics and mammalian cell culture APIs, pH buffers such as phosphate, acetate, and citrate are ever-present, used in preparing culture media and product formulations. However, even microscopic traces of these pH buffers left on contact surfaces like stainless steel trolleys, work tables, or transport trays can cause staining and eventually lead to […]
Cleaning Sticky Residues in Softgel Production Lines

Softgel manufacturers are familiar with the difficulties associated with cleaning gelatin build-up, plasticizers, oils and pigments. Removing these tough residues from stainless steel equipment poses a significant challenge in turnaround times and operational efficiencies. Conventional cleaning techniques like traditional wipes and standard detergents often fail to tackle heat-cured or sticky contaminants effectively. To overcome these […]
Establishing and Validating the Lifecycle of Cleanroom Gowning

Cleanroom environments demand rigorous contamination control with gowning protocols playing a pivotal role in safeguarding product integrity and operator safety. As guided by Annex 1, a critical aspect of gowning management is establishing and validating the lifecycle of cleanroom garments. This process ensures that garments maintain their protective performance throughout their use, balancing safety, comfort […]
How much Ink is being Deposited on a Product’s Surface during Printing: Insights from Ackley Machine Corporation
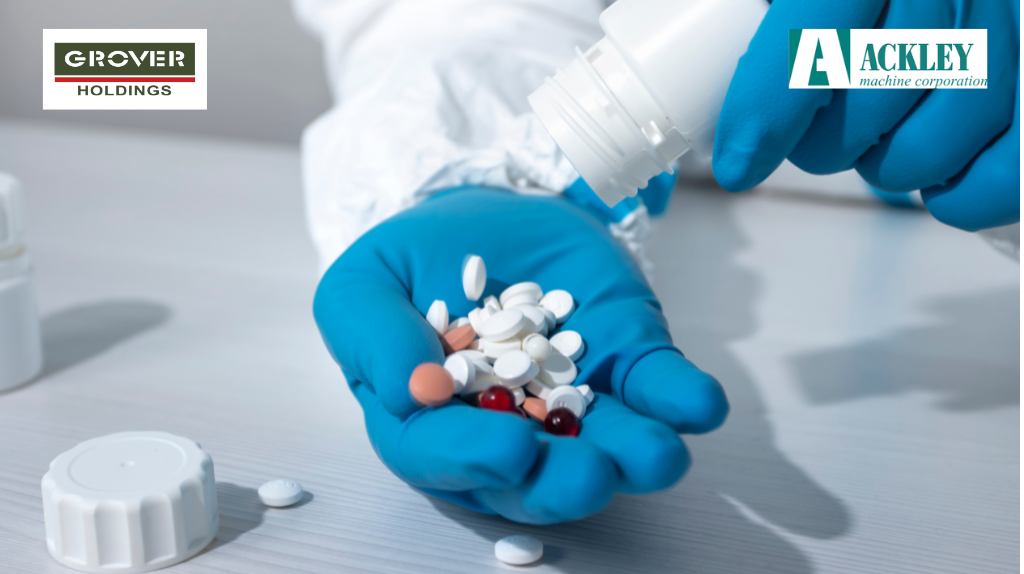
One critical question remains central to precision printing: ‘How much ink is actually being deposited on a product’s surface during the printing process?’ Ackley Machine Corporation, a leader in precision printing technology, has contributed to this growing body of research by analysing ink deposition on various printed products. The Study on Ink Deposition In collaboration […]
Addressing Challenges in Leak Testing for Cold Chain and Terminally Sterilised Products

Leak testing for cold chain and terminally sterilised products presents unique challenges due to factors such as condensation and entrapped moisture, which can compromise the accuracy of traditional detection methods. Challenges Posed by Condensation and Entrapped Moisture Cold chain and terminally sterilized products are particularly susceptible to moisture-related issues during leak testing: 1. Condensation in […]
Ink Printing/Laser marking on Solid Dosage Drug Products-Which is better

Applying identification on solid dosage drug products can be necessary from the point of regulatory compliance, patient safety and brand protection. Identification marking on tablets, capsules and softgels prevents medication errors, ensures traceability and protects against counterfeiting. The choice of marking technology often causes confusion. With large volumes and high speed requirements an added factor […]
The Impact of Physical Attributes of Tablets on Tablet Imprinting with Ink
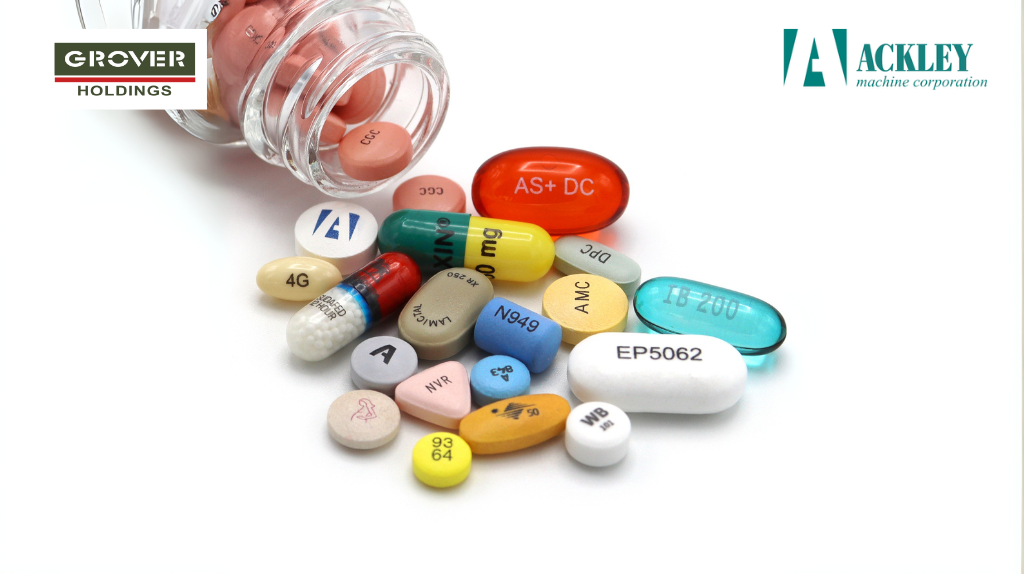
Tablet imprinting is sometimes necessary for ensuring precise identification, brand differentiation and regulatory compliance. However a tablet’s shape, dimensions and surface lubricity significantly impacts the ability to effectively imprint ink logos on them. In practice it is found that many tablets do not lend themselves well to imprinting of logos at high speeds and often […]
The Challenge of Testing Sterile Containers Sealed Under Vacuum for The Presence of Vacuum-Annex 1 compliance

The revised Annex 1 guideline, released in August 2022, includes specific requirements related to container closure integrity. Clause 8.24 states: “Containers sealed under vacuum should be tested for maintenance of vacuum after an appropriate pre-determined period prior to certification/release and during shelf life.” While testing for presence of headspace oxygen is more commonly known and […]
FAQs from PMEC 2024

At the recently concluded PMEC 2024, our booth attracted significant visitor interest by showcasing innovative solutions addressing Annex 1 compliance challenges. The frequently discussed points: 1. Testing for the presence of vacuum in containers sealed under vacuum 2. Cleaning tools and processes to address residues of disinfectants 3. Establishing a validated life cycle for secondary […]
Navigating the Complexity of Lyophilization Cycle for Sensitive Drug Formulations

Image Credits: DALL.E Fill-finish and lyophilization are essential processes in the pharmaceutical industry, particularly for protecting sensitive drug formulations. Fill-finish occurs before lyophilization during which the drug formulation is precisely filled into vials or other primary packaging to ensure dosage accuracy and prevent contamination. Following the fill-finish process, lyophilization significantly improves product stability by removing moisture, prolonging shelf life, […]

