
Image credit: DALL.E
One among these is the deep cleaning, abrasion-resistant MiraWIPE which is an indispensable tool in maintaining the stringent sterility standards required in ISO 5 Clean Rooms.
Navigating the Cleaning Challenge in ISO 5 Pharmaceutical Clean Rooms
The cleaning and maintenance of ISO 5 pharmaceutical Clean Rooms represents a multifaceted challenge, one that includes more than just the eradication of visible contaminants.
The invisible contaminants, primarily in the form of subvisible particles and microorganisms, pose the greatest threat to the sanctity of these controlled environments.
To understand the depth of this challenge, one must consider the various sources of contamination, the stringent regulatory standards that govern Clean Room operations, and the limitations of traditional cleaning methods.
Sources of Contamination
Contamination within Clean Rooms can originate from a wide range of sources.
Human operators, despite being essential to pharmaceutical manufacturing processes, are significant contributors, shedding skin flakes, hair, and emitting bodily microorganisms.
The very materials and equipment used in production processes can also introduce contaminants. Metal particles, dust and fibres from packaging materials, disinfectant residues, fibres from gowning and from cleaning material and last but not the least, the cellular debris after disinfection, further compound the issue.
Regulatory Standards and Implications
The regulatory framework governing ISO 5 pharmaceutical Clean Rooms is underpinned by an array of guidelines and standards.
At the heart of this regulatory landscape are Good Manufacturing Practices (GMP) that guide the production and testing of pharmaceutical products. A key aspect of GMP relevant to Clean Room operations is the stringent control of environmental conditions to prevent contamination of products. This involves detailed specifications for the design, monitoring, and operation of Clean Rooms, including the management of air quality, temperature, humidity, and particulate cleanliness levels.
In addition to GMP, the recent revision of the EU Annex 1 regulations represents a significant development in the regulatory standards governing pharmaceutical Clean Rooms. The revised Annex 1, titled “Manufacture of Sterile Medicinal Products,” guides for a wholistic approach to the Contamination Control strategy which could include enhanced requirements for the design, operation, and monitoring of Clean Room environments. One of the significant aspects highlighted by the revised Annex 1 is the focus on cleaning versus mere disinfection.
The Limitations of Traditional Cleaning Tools
Traditional cleaning tools fall short when it comes to effective performance in ISO 5 pharmaceutical Clean Rooms.
The reasons can be found in the inherent nature of their construction materials, for example, the monofilament smooth round shaped polyester yarn does not offer much abrasion or retention of contaminants and the fused construction of polyester cellulose material sheds particles. Conventional polyester or polyester cellulose wipes for example, often leave behind fibres and particles that can compromise Clean Room integrity. The effectiveness of these tools is also limited by their inability to dislodge, entrap and retain contaminants which are capable of evading capture due to their sticky nature (think disinfectant residues) or being entrenched in the grain of the metal surfaces (think debris of disinfection).
MiraWIPE: Designed for ISO 5 Clean Room Excellence
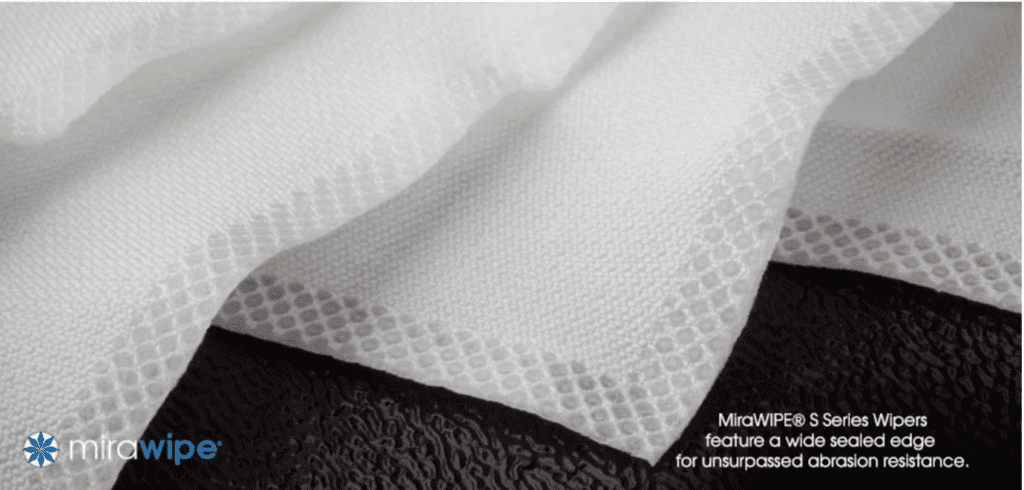

MiraWIPEs from Foamtec are specifically designed to meet the rigorous demands of ISO 5 pharmaceutical Clean Room environments.
Crafted from a high-density, super smooth woven polyester/nylon fabric, MiraWIPE is designed to minimize the release of submicron particulates, addressing a critical challenge in Clean Room maintenance. It practically eliminates the shedding of particles larger than 0.50 microns from the wipe’s surface during heavy-duty cleaning tasks, a common issue with conventional cleaning materials.
One of the unique attributes of MiraWIPE is abrasion resistance. It’s non-stretch fabric, complemented by ultrasonic wide sealed edges that prevent splitting,eliminates a major source of fibre generation. This design innovation ensures that the integrity of the wipe is maintained throughout its use, further reinforcing the cleanliness of the environment.
Moreover, the MiraWipe’s non-symmetrical structure and star shaped filament is engineered to dislodge stubbornly adhered contaminants. This unique split filament design increases surface area that allows access to the microscopic troughs in the surfaces for effective entrapment and removal of residual contaminants from product contact surfaces and components.
MiraWIPE’s compatibility with ISO Class 5 cleanrooms is ensured through a meticulous laundering and packing process. Each lot of MiraWIPE undergoes rigorous testing and certification by LPC, Helmke drum, and optical test methods. This ensures the control of particle contamination from submicron up to 500 microns. All wipers are double bagged to aid clean room entry protocols.
Summing Up:
Implementing the MiraWipe as an additional cleaning tool for a final wipe down at the end of a batch and prior to disinfection of the surfaces under ISO 5 provides a very effective cleaning step as guided by the revised Annex 1 regulations. For more information on MiraWIPE, please reach out to us on [email protected].
Credits
Foamtec International, Wilshire Contamination Control Division.
Alsico Iberia S.L.(formerly known as Vestilab)

