
Image credits: Foamtec
When it comes to the complex world of manufacturing sterile pharmaceuticals, maintaining contamination control in Clean Room environments becomes paramount.
The Common Challenge in Validating Clean Room Surfaces
Good Manufacturing Practice (GMP) procedures mandate that operators frequently sanitize the work surfaces at the beginning and end of their shifts, as well as after any interventions.
However, a significant challenge arises due to the inherent physical characteristics of these work surfaces. Given that they are often stainless-steel surfaces, it is very common for minute particles and residues to easily go unnoticed. This is compounded by the fact that many such contaminants are virtually invisible to the naked eye. Many a times, work surfaces that visually appear clean can have microscopic contaminants that can compromise sterile products.
Such unintended oversights can have significant consequences, ranging from product recalls to serious health hazards for patients. Additionally, the financial implications of such lapses, both in terms of recall costs and potential legal liabilities, can be significant.
Finally, a more serious challenge that awaits Clean Room operators is not just capturing these contaminants but also understanding their source such that Corrective and Preventive Actions (CAPAs) can be taken to their logical conclusions.
Enhancing Clean Room Contamination Control with_PolyCheck Wipes
The black coloured PolyCHECK inspection wipes bring a paradigm shift in how cleaning itself is validated.
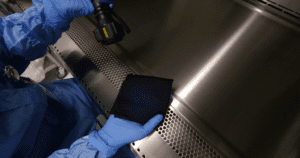
PolyCHECK’s unique design uses a highly charged black fabric that helps cleaning validation engineers with unparalleled visibility into difficult-to-observe white residues on stainless steel equipment. Enhanced clarity like this significantly enhances the verification and validation processes for cleaning, making sure that no traces of contamination are left unchecked..
The PolyCHECK wipes can becombined with a UV inspection torch to take cleaning verification a step further. This strategic pairing facilitates the easy detection of organic particles that emit fluorescence under UV light when applied to the black fabric. This groundbreaking method is a game-changer in the validation of cleanliness, raising standards and precision.
But the brilliance of the PolyCHECK wipes doesn’t just stop at detection. The contaminants can be further analysed using techniques like Scanning Electron Microscopy/Energy Dispersive X-ray (SEM/EDX) and Fourier Transform Infrared (FTIR) analysis.
These techniques allow for detailed characterization of the particles, including their elemental composition and molecular structure. Quality engineers can then identify the material and thereby the source of visible particles and implement targeted root cause corrective actions.
This proactive approach leads to improved quality control and a significant reduction in particle-related issues, ensuring the safety and efficacy of parenteral drug products.
To learn how PolyCHECK wipes can help your Clean Rooms achieve impeccable cleanliness, get in touch with us at vivek@groverholdings.com.

